Providing pharmaceutical solutions




A VISION FOR RESEARCH AND PRODUCT DEVELOPMENT
Innovating new ways to address a variety of eye care challenges is an Opto-Pharm passion shared by all our scientists and lab technicians, who regularly develop market-leading products aimed at exceeding the needs of consumers.
We partner with a variety of entities to develop the ideal solutions with their unique specification and market needs in mind.


TESTING SOLUTIONS
Our laboratory services support GMP (Good Manufacturing Practice) and CMC (Chemistry, Manufacturing and Controls) requirements, and cover early development characterisation, formulation support analysis, stability testing, method development and method validation through to GMP batch release testing.


MANUFACTURING
Quality is paramount. That is why we utilise state-of-the-art machinery and production processes throughout our manufacturing departments.
BFS (Blow-Fill-Seal) technology is an automated, aseptic packaging process where plastic containers are blow-molded, filled and sealed in one continuous, protected operation.
This process eliminates all contact with foreign substances ensuring quality in a controlled environment.


QUALITY MANAGEMENT
We are a single-source provider for most of our clientele, which means rigorous quality management is paramount. That is why we have dedicated decades to the set-up of a quality department to guide the manufacturing of our contact lens care and sterile pharmaceutical solutions. Our GMP certified plant and laboratory perform a comprehensive menu of routine and customised safety and efficacy tests, providing chemistry, manufacturing and protocols (CMC) analytical services that meet the exacting requirements of regulatory authorities worldwide.
OUR TECHNOLOGY — THE BFS PROCESS
EXTRUSION
A plastic tube extruded from plastic granules is fed into the opened blow mould.
MOLDING
The main mold closes, sealing the base in the process. The blow mandrel is lowered onto the neck of the mold, then inflates the parison into a hollowed container with compressed air.
FILLING
A mandrel fills the container with the product, measured by a precise dosing system.
SEALING
The mandrel is removed, the head closes, and the required closure is formed via vacuum.
DEMOLDING
The blow mold opens, allowing the container to depart the machine. A conveyer system takes the container to the next processing stage, allowing the next cycle to commence.
3D VISUALISATION OF THE Blow-Fill-Seal process
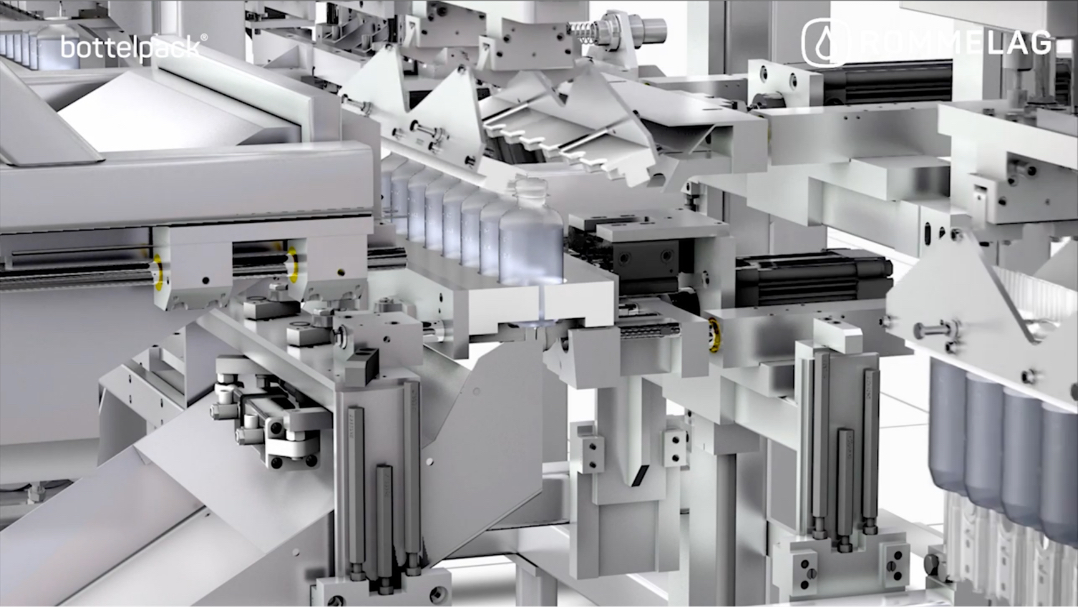
Video by: Rommelag Group of Companies
BFS technology is recognised in the liquid pharmaceutical industry as an efficient, advanced aseptic processing technology. Our high-efficiency, high-output BFS machines have the capability to produce millions of bottles and ampoules to meet global demand.
BFS technology trumps traditional aseptic filling techniques with superior levels of quality assurance. Tried and tested, the technology boasts an excellent track record of numerous new product launches in the field of ophthalmic and irrigation products.
You’ve seen how we work. Now take a look at what we make.



